Welcome to the polyatomic ions WS answer key, where you’ll embark on a journey into the fascinating realm of complex ions. Dive into the intricacies of their structure, nomenclature, and reactivity, and unlock a deeper understanding of chemistry.
From the basics of polyatomic ion formation to their role in chemical reactions, this guide will illuminate the mysteries surrounding these essential chemical entities.
Definition and Composition of Polyatomic Ions
Polyatomic ions are electrically charged species composed of two or more atoms covalently bonded together. These ions carry a net charge and behave as a single unit within chemical reactions.
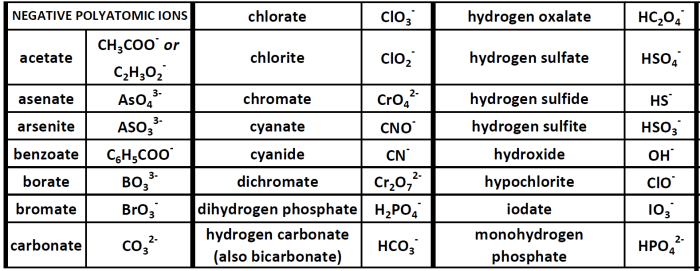
Common polyatomic ions include:
- Hydroxide (OH –)
- Carbonate (CO 32-)
- Sulfate (SO 42-)
- Nitrate (NO 3–)
- Phosphate (PO 43-)
Structure and Bonding
Polyatomic ions are held together by covalent bonds, where electrons are shared between the atoms. The structure of these ions can vary depending on the specific atoms involved and the number of electrons shared.
For example, the hydroxide ion (OH –) consists of one oxygen atom and one hydrogen atom covalently bonded together. The oxygen atom has two lone pairs of electrons, while the hydrogen atom has one electron. The covalent bond between the oxygen and hydrogen atoms results in a net negative charge on the ion.
Nomenclature of Polyatomic Ions: Polyatomic Ions Ws Answer Key
Polyatomic ions are ions composed of more than one atom. They have a specific set of rules for naming them.
Use of Prefixes and Suffixes
The name of a polyatomic ion includes a prefix and a suffix. The prefix indicates the number of atoms in the ion, while the suffix indicates the charge of the ion.
- Prefixes:mono-, di-, tri-, tetra-, penta-, hexa-, hepta-, octa-, nona-, deca-.
- Suffixes:-ide (for negative ions), -ate (for negative ions with more oxygen atoms), -ite (for negative ions with fewer oxygen atoms).
Examples of Polyatomic Ions with Different Charges
- Carbonate (CO32-): A polyatomic ion with three oxygen atoms and one carbon atom. It has a charge of -2.
- Sulfate (SO42-): A polyatomic ion with four oxygen atoms and one sulfur atom. It has a charge of -2.
- Nitrate (NO3–): A polyatomic ion with three oxygen atoms and one nitrogen atom. It has a charge of -1.
Properties of Polyatomic Ions
Polyatomic ions are groups of atoms that carry an electric charge. They exhibit unique physical and chemical properties that differ from those of individual atoms or monatomic ions.The charge and structure of polyatomic ions significantly influence their properties. Ions with a high charge tend to be more reactive than those with a low charge.
The shape and size of the ion also affect its reactivity and solubility. For example, the sulfate ion (SO42-) is a highly charged, tetrahedral ion that is highly soluble in water. In contrast, the carbonate ion (CO32-) is a moderately charged, planar ion that is less soluble in water.Polyatomic
ions can exhibit a wide range of properties. Some polyatomic ions, such as the hydroxide ion (OH-), are strong bases. Others, such as the ammonium ion (NH4+), are weak acids. Some polyatomic ions, such as the cyanide ion (CN-), are toxic.
Others, such as the nitrate ion (NO3-), are relatively harmless.The properties of polyatomic ions are important to understand in various fields of chemistry, including inorganic chemistry, biochemistry, and environmental chemistry. By understanding the properties of polyatomic ions, chemists can better understand the behavior of chemical compounds and predict their reactivity.
Physical Properties
The physical properties of polyatomic ions include their color, shape, and solubility. The color of a polyatomic ion is determined by the electronic structure of the ion. The shape of a polyatomic ion is determined by the arrangement of the atoms within the ion.
Polyatomic ions can be a bit tricky to keep track of, but luckily there are plenty of resources available to help you out. If you’re looking for a comprehensive guide to polyatomic ions, be sure to check out our answer key.
And if you’re struggling with geometry, we also have a great resource for you: the big ideas geometry answer key . This answer key will help you master all the concepts you need to know for your geometry class.
The solubility of a polyatomic ion is determined by the strength of the electrostatic forces between the ion and water molecules.
Chemical Properties
The chemical properties of polyatomic ions include their reactivity, acidity, and basicity. The reactivity of a polyatomic ion is determined by the charge and size of the ion. The acidity of a polyatomic ion is determined by the ability of the ion to donate a proton.
The basicity of a polyatomic ion is determined by the ability of the ion to accept a proton.
Reactions Involving Polyatomic Ions
Polyatomic ions are charged chemical species composed of two or more atoms covalently bonded together. They play significant roles in various chemical reactions, particularly in acid-base reactions and redox reactions.
Participation in Chemical Reactions
Polyatomic ions participate in chemical reactions by donating or accepting electrons, forming ionic bonds with other ions. They can undergo various types of reactions, including:
- Precipitation reactions:Polyatomic ions can form insoluble precipitates when combined with appropriate cations. For example, the reaction between barium ions (Ba 2+) and sulfate ions (SO 42-) forms barium sulfate (BaSO 4), a white precipitate.
- Acid-base reactions:Polyatomic ions can act as acids or bases, depending on their ability to donate or accept protons (H +). For example, the hydrogen carbonate ion (HCO 3–) can donate a proton to act as an acid, while the hydroxide ion (OH –) can accept a proton to act as a base.
- Redox reactions:Polyatomic ions can participate in redox reactions, where one ion undergoes oxidation (loss of electrons) while another undergoes reduction (gain of electrons). For example, the permanganate ion (MnO 4–) can undergo reduction to form the manganate ion (MnO 42-), while the dichromate ion (Cr 2O 72-) can undergo oxidation to form the chromate ion (CrO 42-).
Role in Acid-Base Reactions
Polyatomic ions play a crucial role in acid-base reactions by acting as either acids or bases. Acids are substances that donate protons (H +), while bases are substances that accept protons. Polyatomic ions can form both weak and strong acids and bases.
For example, the hydrogen carbonate ion (HCO 3–) is a weak acid that can donate a proton to form carbonic acid (H 2CO 3), while the hydroxide ion (OH –) is a strong base that can accept a proton to form water (H 2O).
Role in Redox Reactions, Polyatomic ions ws answer key
Polyatomic ions can also participate in redox reactions, where one ion undergoes oxidation (loss of electrons) while another undergoes reduction (gain of electrons). Redox reactions involve the transfer of electrons between reactants.
For example, the permanganate ion (MnO 4–) can undergo reduction to form the manganate ion (MnO 42-), while the dichromate ion (Cr 2O 72-) can undergo oxidation to form the chromate ion (CrO 42-).
Applications of Polyatomic Ions
Polyatomic ions play crucial roles in various fields, including industry, medicine, and environmental science. Their unique properties and reactivity make them indispensable for numerous applications.
Industrial Applications
- Electroplating: Polyatomic ions are used to coat metals with a protective layer, enhancing their durability and appearance. For instance, chromate ions (CrO 42-) are used in chrome plating, providing a shiny and corrosion-resistant surface.
- Batteries: Polyatomic ions are key components in batteries, such as the lead-acid battery (PbO 2and PbSO 4) and the nickel-cadmium battery (NiOOH and Cd).
- Fertilizers: Polyatomic ions, such as nitrate (NO 3–), phosphate (PO 43-), and ammonium (NH 4+), are essential nutrients for plants. Fertilizers containing these ions enhance crop yield and soil fertility.
Medical Applications
- Radiopharmaceuticals: Polyatomic ions, like iodide (I –) and technetium (TcO 4–), are used as radioactive tracers in medical imaging techniques, such as thyroid scans and bone scans.
- Antibiotics: Polyatomic ions, including penicillin (C 16H 18N 2O 4S –) and erythromycin (C 37H 67NO 13+), are effective antimicrobial agents used to treat bacterial infections.
- Antacids: Polyatomic ions, such as bicarbonate (HCO 3–) and hydroxide (OH –), are used in antacids to neutralize stomach acid and relieve heartburn.
Environmental Applications
- Water Treatment: Polyatomic ions, like sulfate (SO 42-) and chloride (Cl –), are used as coagulants and disinfectants in water treatment plants, removing impurities and ensuring water safety.
- Wastewater Treatment: Polyatomic ions, including nitrate (NO 3–) and phosphate (PO 43-), are removed from wastewater to prevent eutrophication, a process that can lead to algal blooms and oxygen depletion.
- Soil Remediation: Polyatomic ions, such as phosphate (PO 43-) and sulfate (SO 42-), are used to immobilize heavy metals in contaminated soils, reducing their bioavailability and potential environmental hazards.
Clarifying Questions
What is a polyatomic ion?
A polyatomic ion is a group of atoms that carries a net electrical charge and behaves as a single unit within a compound.
How do I name polyatomic ions?
Polyatomic ions are named using prefixes that indicate the number of atoms in the ion and suffixes that indicate the charge of the ion.
What are some examples of polyatomic ions?
Common polyatomic ions include hydroxide (OH-), sulfate (SO42-), and nitrate (NO3-).