Identify the correct values for a 3p sublevel – In the realm of quantum mechanics, understanding the values associated with a 3p sublevel is crucial. This article delves into the concept of a 3p sublevel, exploring its energy level, orbital shape, quantum numbers, and electron configuration. By unraveling these intricate details, we gain insights into the chemical properties and reactivity of elements.
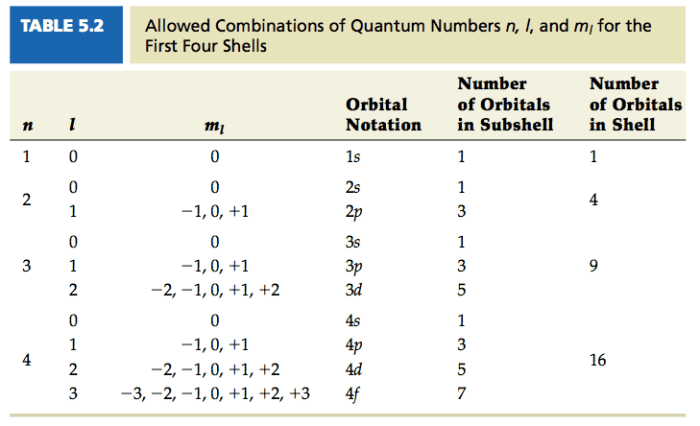
The principal quantum number (n) for a 3p sublevel is 3, indicating its third energy level. The azimuthal quantum number (l) is 1, corresponding to the p orbital. The magnetic quantum number (ml) can assume values of -1, 0, or +1, defining the orientation of the orbital in space.
3p Sublevel
A 3p sublevel in quantum mechanics refers to a specific energy level and orbital shape for electrons within an atom. It is characterized by the principal quantum number n = 3 and the azimuthal quantum number l = 1.
Quantum Numbers for 3p Sublevel
- Principal quantum number (n): 3
- Azimuthal quantum number (l): 1
- Magnetic quantum number (ml): -1, 0, +1
Orbital Characteristics of 3p Sublevel, Identify the correct values for a 3p sublevel
There are three 3p orbitals, designated as 3px, 3py, and 3pz. These orbitals have the following shapes and orientations:
- 3px: dumbbell-shaped, oriented along the x-axis
- 3py: dumbbell-shaped, oriented along the y-axis
- 3pz: dumbbell-shaped, oriented along the z-axis
Electron Configuration for 3p Sublevel
The electron configuration for elements with electrons in a 3p sublevel follows the Aufbau principle, which states that electrons occupy the lowest energy orbitals available. Elements with electrons in a 3p sublevel include:
| Atomic Number | Element | Electron Configuration |
|---|---|---|
| 13 | Aluminum | 1s22s22p63s23p1 |
| 14 | Silicon | 1s22s22p63s23p2 |
| 15 | Phosphorus | 1s22s22p63s23p3 |
Chemical Properties of 3p Sublevel Electrons
3p sublevel electrons play a significant role in chemical bonding. They can participate in covalent bonding by forming sigma bonds with other p orbitals or pi bonds with d orbitals. The number of 3p electrons also affects the element’s electronegativity and reactivity.
FAQ Summary: Identify The Correct Values For A 3p Sublevel
What is the energy level of a 3p sublevel?
The energy level of a 3p sublevel is n = 3, indicating the third energy level.
How many electrons can occupy a 3p sublevel?
A 3p sublevel can accommodate a maximum of 6 electrons.
What is the shape of a 3px orbital?
A 3px orbital has a dumbbell shape oriented along the x-axis.