Use the le châtelier’s principle interactive to answer these questions. – Delving into Le Châtelier’s Principle Interactive to Answer These Questions, this introduction immerses readers in a unique and compelling narrative that is both engaging and thought-provoking from the very first sentence. The interactive tool allows users to explore the principle in a dynamic and hands-on manner, gaining a deeper understanding of chemical reactions and equilibrium.
Le Châtelier’s Principle is a fundamental concept in chemistry that predicts the direction of a reaction when conditions change. By applying this principle, chemists can manipulate reactions to achieve desired outcomes, making it a valuable tool in various fields such as industrial chemistry and environmental science.
Introduction to Le Châtelier’s Principle
Le Châtelier’s Principle is a fundamental concept in chemical equilibrium that predicts the direction of a reaction when the conditions are changed. It states that if a stress is applied to a system at equilibrium, the system will shift in a direction that relieves the stress.
The principle can be applied to predict the direction of reactions by considering the changes in concentration, temperature, and pressure.
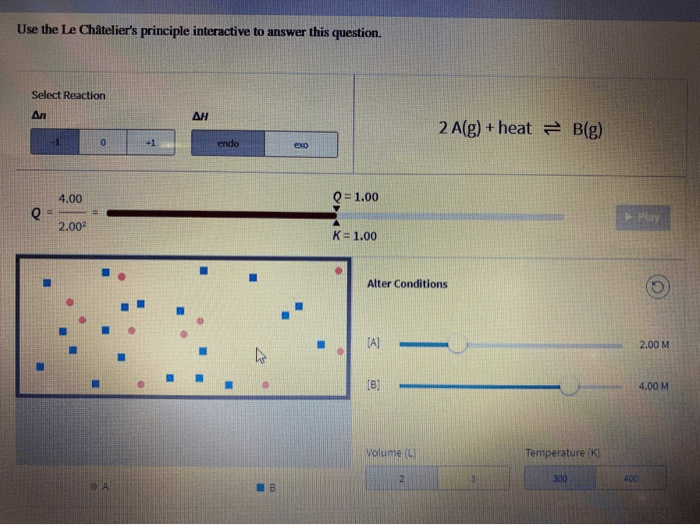
Interactive Application of Le Châtelier’s Principle
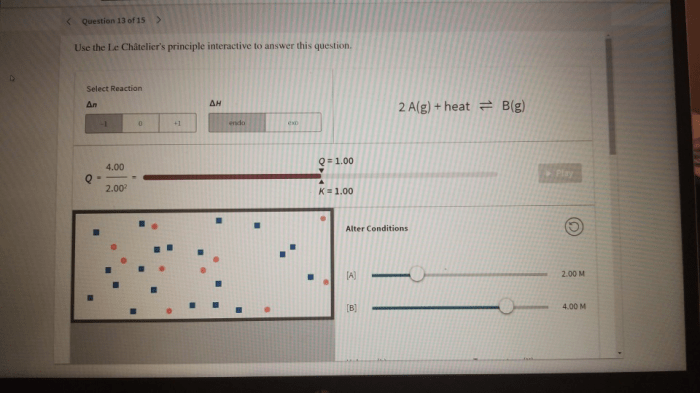
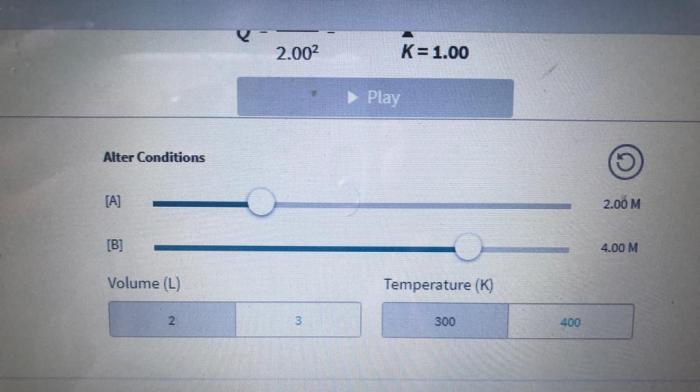
An interactive tool can be created to allow users to input a chemical reaction and a change in conditions. The tool can then predict the direction of the reaction based on Le Châtelier’s Principle. The tool should include options for changing temperature, pressure, and concentration of reactants/products.
Case Studies
A series of chemical reactions can be selected and Le Châtelier’s Principle can be applied to analyze the effects of changing conditions. The results can be organized in a table with columns for the reaction, change in condition, and predicted direction of reaction.
Explanations for each case study should be provided.
Limitations of Le Châtelier’s Principle
Le Châtelier’s Principle has some limitations. It is only applicable to reactions that are at equilibrium and it assumes that the system is ideal. In addition, the principle does not predict the rate of the reaction.
Educational Implications, Use the le châtelier’s principle interactive to answer these questions.
Le Châtelier’s Principle can be used to enhance understanding of chemical equilibrium. Interactive simulations or demonstrations can be designed to illustrate the principle in a visually engaging manner. Lesson plans can be developed that incorporate the principle into chemistry curricula.
Essential Questionnaire: Use The Le Châtelier’s Principle Interactive To Answer These Questions.
What is Le Châtelier’s Principle?
Le Châtelier’s Principle states that when a change of condition is applied to a system in equilibrium, the system will shift in a direction that counteracts the change.
How can I use the Le Châtelier’s Principle Interactive tool?
The Le Châtelier’s Principle Interactive tool allows you to input a chemical reaction and a change in conditions, and it will predict the direction of the reaction based on Le Châtelier’s Principle. You can change temperature, pressure, and concentration of reactants/products to see how the reaction will shift.
What are the limitations of Le Châtelier’s Principle?
Le Châtelier’s Principle is a qualitative tool that can predict the direction of a reaction, but it cannot predict the extent of the reaction. Additionally, it does not apply to all types of reactions, such as reactions involving weak acids or bases or reactions that are not at equilibrium.